Stem cells: What they are and what they do
Stem cells: What they are and what they do
You've heard about stem cells in the news, and perhaps you've wondered if they might help you or a loved one with a serious disease. Here are some answers to frequently asked questions about stem cells.
What are stem cells?
Stem cells are a special type of cells that have two important properties. They are able to make more cells like themselves. That is, they self-renew. And they can become other cells that do different things in a process known as differentiation. Stem cells are found in almost all tissues of the body. And they are needed for the maintenance of tissue as well as for repair after injury.
Depending on where the stem cells are, they can develop into different tissues. For example, hematopoietic stem cells reside in the bone marrow and can produce all the cells that function in the blood. Stem cells also can become brain cells, heart muscle cells, bone cells or other cell types.
There are various types of stem cells. Embryonic stem cells are the most versatile since they can develop into all the cells of the developing fetus. The majority of stem cells in the body have fewer abilities to give rise to cells and may only help maintain and repair the tissues and organs in which they reside.
No other cell in the body has the natural ability to generate new cell types.
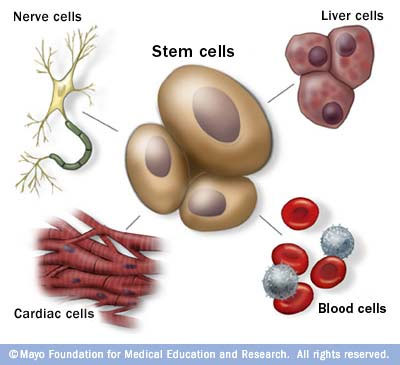
Stem cells are the body's master cells. All other cells arise from stem cells, including blood cells, nerve cells and other cells.
Why is there such an interest in stem cells?
Researchers are studying stem cells to see if they can help to:
- Increase understanding of how diseases occur. By watching stem cells mature into cells in bones, heart muscle, nerves, and other organs and tissue, researchers may better understand how diseases and conditions develop.
Generate healthy cells to replace cells affected by disease (regenerative medicine). Stem cells can be guided into becoming specific cells that can be used in people to regenerate and repair tissues that have been damaged or affected by disease.
People who might benefit from stem cell therapies include those with leukemia, Hodgkin disease, non-Hodgkin lymphoma and some solid tumor cancers. Stem cell therapies also might benefit people who have aplastic anemia, immunodeficiencies and inherited conditions of metabolism.
Stem cells are being studied to treat type 1 diabetes, Parkinson's disease, amyotrophic lateral sclerosis, heart failure, osteoarthritis and other conditions.
Stem cells may have the potential to be grown to become new tissue for use in transplant and regenerative medicine. Researchers continue to advance the knowledge on stem cells and their applications in transplant and regenerative medicine.
-
Test new drugs for safety and effectiveness. Before giving drugs in development to people, researchers can use some types of stem cells to test the drugs for safety and quality. This type of testing may help assess drugs in development for toxicity to the heart.
New areas of study include the effectiveness of using human stem cells that have been programmed into tissue-specific cells to test new drugs. For the testing of new drugs to be accurate, the cells must be programmed to acquire properties of the type of cells targeted by the drug. Techniques to program cells into specific cells are under study.
Where do stem cells come from?
There are several sources of stem cells:
-
Embryonic stem cells. These stem cells come from embryos that are 3 to 5 days old. At this stage, an embryo is called a blastocyst and has about 150 cells.
These are pluripotent (ploo-RIP-uh-tunt) stem cells, meaning they can divide into more stem cells or can become any type of cell in the body. This allows embryonic stem cells to be used to regenerate or repair diseased tissue and organs.
- Adult stem cells. These stem cells are found in small numbers in most adult tissues, such as bone marrow or fat. Compared with embryonic stem cells, adult stem cells have a more limited ability to give rise to various cells of the body.
-
Adult cells altered to have properties of embryonic stem cells. Scientists have transformed regular adult cells into stem cells using genetic reprogramming. By altering the genes in the adult cells, researchers can make the cells act similarly to embryonic stem cells. These cells are called induced pluripotent stem cells (iPSCs).
This new technique may allow use of reprogrammed cells instead of embryonic stem cells and prevent immune system rejection of the new stem cells. However, scientists don't yet know whether using altered adult cells will cause adverse effects in humans.
Researchers have been able to take regular connective tissue cells and reprogram them to become functional heart cells. In studies, animals with heart failure that were injected with new heart cells had better heart function and survival time.
-
Perinatal stem cells. Researchers have discovered stem cells in amniotic fluid as well as umbilical cord blood. These stem cells can change into specialized cells.
Amniotic fluid fills the sac that surrounds and protects a developing fetus in the uterus. Researchers have identified stem cells in samples of amniotic fluid drawn from pregnant women for testing or treatment — a procedure called amniocentesis.
Why is there controversy about using embryonic stem cells?
Embryonic stem cells are taken from early-stage embryos — a group of cells that forms when eggs are fertilized with sperm at an in vitro fertilization clinic. Because human embryonic stem cells are taken from human embryos, several questions have been raised about the ethics of embryonic stem cell research.
The National Institutes of Health created guidelines for human stem cell research in 2009. The guidelines define embryonic stem cells and how they may be used in research and include recommendations for the donation of embryonic stem cells. Also, the guidelines state that embryonic stem cells from embryos created by in vitro fertilization can be used only when the embryo is no longer needed.
Where do these embryos come from?
The embryos being used in embryonic stem cell research come from eggs that were fertilized at in vitro fertilization clinics but never implanted in women's uteruses. The stem cells are donated with informed consent from donors. The stem cells can live and grow in special solutions in test tubes or petri dishes in laboratories.
Why can't researchers use adult stem cells instead?
Progress in cell reprogramming and the formation of iPSCs has greatly enhanced research in this field. However, reprogramming is an inefficient process. When possible, iPSCs are used instead of embryonic stem cells since this avoids the ethical issues about use of embryonic stem cells that may be morally objectionable for some people.
Although research into adult stem cells is promising, adult stem cells may not be as versatile and durable as are embryonic stem cells. Adult stem cells may not be able to be manipulated to produce all cell types, which limits how adult stem cells can be used to treat diseases.
Adult stem cells are also more likely to contain irregularities due to environmental hazards, such as toxins, or from errors acquired by the cells during replication. However, researchers have found that adult stem cells are more adaptable than was first thought.
What are stem cell lines, and why do researchers want to use them?
A stem cell line is a group of cells that all descend from a single original stem cell and are grown in a lab. Cells in a stem cell line keep growing but don't become specialized cells. Ideally, they remain free of genetic defects and continue to create more stem cells. Clusters of cells can be taken from a stem cell line and frozen for storage or shared with other researchers.
What is stem cell therapy (regenerative medicine), and how does it work?
Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply.
Researchers grow stem cells in a lab. These stem cells are manipulated to specialize into specific types of cells, such as heart muscle cells, blood cells or nerve cells.
The specialized cells can then be implanted into a person. For example, if the person has heart disease, the cells could be injected into the heart muscle. The healthy transplanted heart muscle cells could then contribute to repairing the injured heart muscle.
Researchers have already shown that adult bone marrow cells guided to become heart-like cells can repair heart tissue in people, and more research is ongoing.
Have stem cells already been used to treat diseases?
Yes. Doctors have performed stem cell transplants, also known as bone marrow transplants, for many decades. In hematopoietic stem cell transplants, stem cells replace cells damaged by chemotherapy or disease or serve as a way for the donor's immune system to fight some types of cancer and blood-related diseases. Leukemia, lymphoma, neuroblastoma and multiple myeloma often are treated this way. These transplants use adult stem cells or umbilical cord blood.
Researchers are testing adult stem cells to treat other conditions, including some degenerative diseases such as heart failure.
What are the potential problems with using embryonic stem cells in humans?
For embryonic stem cells to be useful, researchers must be certain that the stem cells will differentiate into the specific cell types desired.
Researchers have discovered ways to direct stem cells to become specific types of cells, such as directing embryonic stem cells to become heart cells. Research is ongoing in this area.
Embryonic stem cells also can grow irregularly or specialize in different cell types spontaneously. Researchers are studying how to control the growth and development of embryonic stem cells.
Embryonic stem cells also might trigger an immune response in which the recipient's body attacks the stem cells as foreign invaders, or the stem cells might simply fail to function as expected, with unknown consequences. Researchers continue to study how to avoid these possible complications.
What is therapeutic cloning, and what benefits might it offer?
Therapeutic cloning, also called somatic cell nuclear transfer, is a way to create versatile stem cells independent of fertilized eggs. In this technique, the nucleus is removed from an unfertilized egg. This nucleus contains the genetic material. The nucleus also is removed from the cell of a donor.
This donor nucleus is then injected into the egg, replacing the nucleus that was removed, in a process called nuclear transfer. The egg is allowed to divide and soon forms a blastocyst. This process creates a line of stem cells that is genetically identical to the donor's cells — in essence, a clone.
Some researchers believe that stem cells derived from therapeutic cloning may offer benefits over those from fertilized eggs because cloned cells are less likely to be rejected once transplanted back into the donor. And it may allow researchers to see exactly how a disease develops.
Has therapeutic cloning in people been successful?
No. Researchers haven't been able to successfully perform therapeutic cloning with humans despite success in a number of other species.
Researchers continue to study the potential of therapeutic cloning in people.
Last Updated Mar 23, 2024
© 2024 Mayo Foundation for Medical Education and Research (MFMER). All rights reserved. Terms of Use